Influenza Surveillance
Seasonal influenza vaccine safety surveillance studies took place between 2009-2019 and 2022-today
From 2009 to 2011, CANVAS examined the short and long term safety of the 2009 AS03 adjuvanted monovalent pandemic vaccine among health care workers at a tertiary care children’s and women’s hospitals, and assessed the acceptability and feasibility of self-reporting for adverse events following influenza immunization through an active web-based electronic survaillance.
The high response rate and acceptability of the online report method suggested that the web-based self-reporting for adverse event following immunization had the potential for rapid assessments of AEFI in mass or new immunization programs.
Since 2012, the network has extended its coverage to the general population including children to provide real-time safety information to public health authorities.
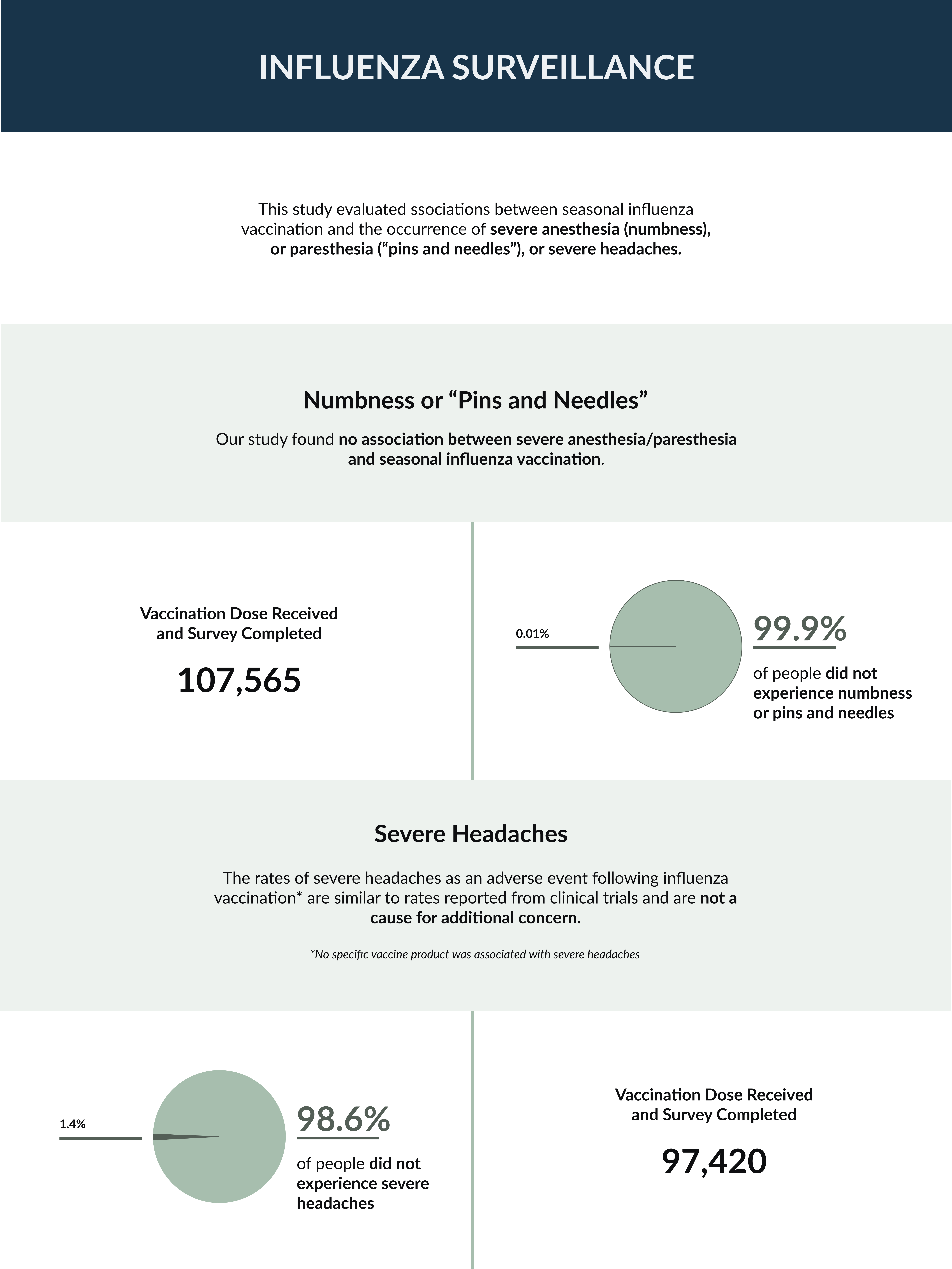
-
Background: https://pubmed.ncbi.nlm.nih.gov/25361187/
-
Infographic data: https://pubmed.ncbi.nlm.nih.gov/32229052/
